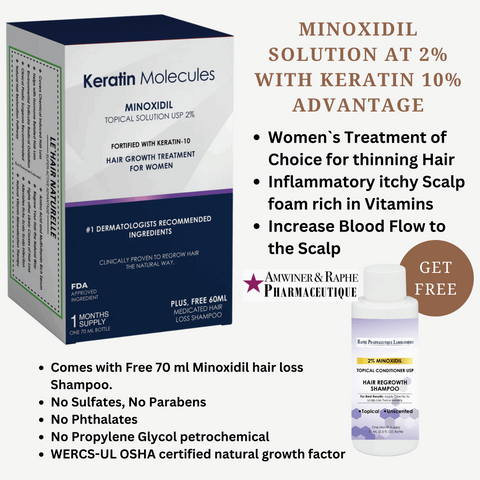
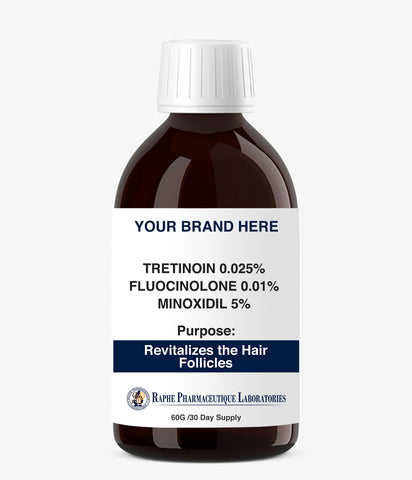
For Healthcare professionals, pharmaceutical product distributors of generic drug products, online pharmacy stores click here to be transferred to our Prescription Drug Facility website
Click here for the over-the-counter products we manufacture. Our OTC drug products are produced under cGMP manufacturing standards and regulations with professionally trained scientists.
Whether you have a small project or a large formulation project, we offer flexible solutions that enable you to move your brand through each phase with efficiency and speed. Our early development solutions enable you to:
- Overcome complex formulation challenges: bioavailability solubility, stability, process, and navigating a complex regulatory environment.
- Build success with your and enable commercial distribution to stores
- Shorten timelines to get to market quicker
- Ensure a robust supply chain within a global network
We conduct early stages of ingredient tests to define and establish product requirements and specifications to meet the needs of your end-users. Verification and validation testing are essential to confirm that the product you end up using meets those specifications and its intended use.




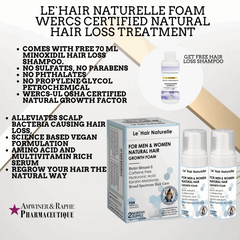

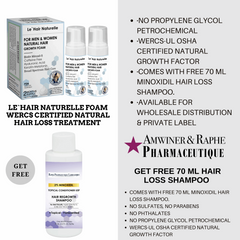
 Pin it
Pin it